iPlexus Dashboard
Developer: Innoplexus Consulting Services Private Limited
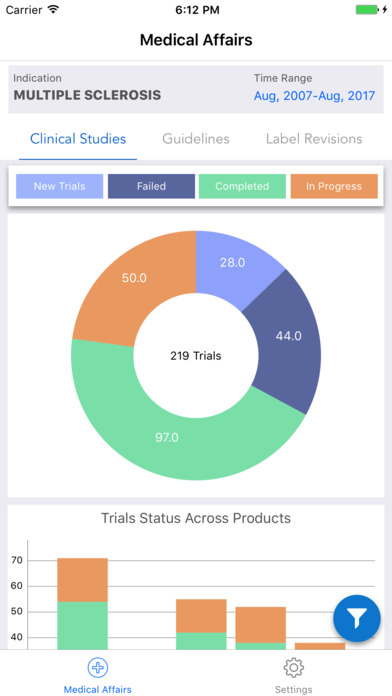
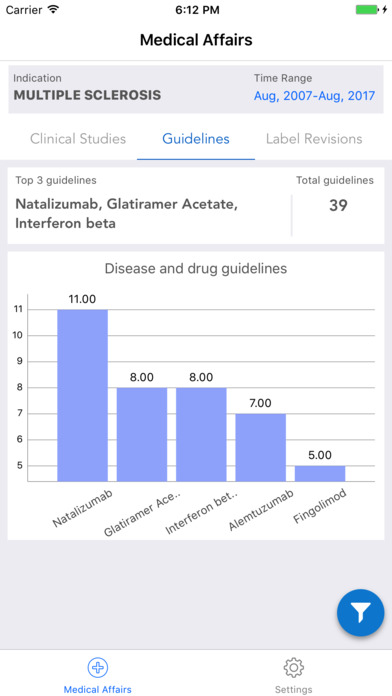
iPlexus Medical Affairs is a dashboard giving its users an at a glance information on the clinical trials status for all the interventions of user’s interest. The present dashboard discusses about the considerably top 10 interventions under clinical trials which can treat ‘Multiple Sclerosis’. As the users of this dashboard are mainly understood to be Medical Affairs personnel, it provides a gist to them on the clinical trials, disease and drug guidelines, and the label revisions of the drugs
iPlexus Regulatory Affairs is a dashboard giving its users an at a glance information on the latest regulatory updates of major regulatory boards, disease and drug guidelines, and drug launches across regulatory bodies. The present dashboard discusses about the indication ‘Multiple Sclerosis’ and the related latest regulatory updates. As the users of this dashboard are mainly understood to be Regulatory Affairs personnel to develop and execute regulatory strategy to ensure the collective efforts of the drug development team results in a product is approvable by global regulators but is also different from the competitors’ market in a way. The information on our dashboard will help the regulatory affairs personnel to get all the information at one stop while the data is curated, analysed and made visually appealing.